BC Platforms launches next generation trusted research environment on AWS Marketplace
BC Mosaic, our trusted research environment, now on AWS Marketplace for secure, AI-powered federated data analysis.
Innovative solutions spanning global real-world data (RWD), analytics, and technology enable confident decision-making and faster innovation.
Whether you’re in clinical development, preparing for launch, or assessing post-approval impact, tap into the world’s most extensive patient data, analytics, and tech-driven solutions to inform evidence-based decisions every step of the way.
Draw on data from our proprietary catchment that includes 187 million+ patients from healthcare and research organizations around the world – Europe, Japan, APAC, and the U.S. We’ll apply your research objectives to identify, ingest, harmonize, and integrate the right data sources to ensure your projects are set up for success from Day One.
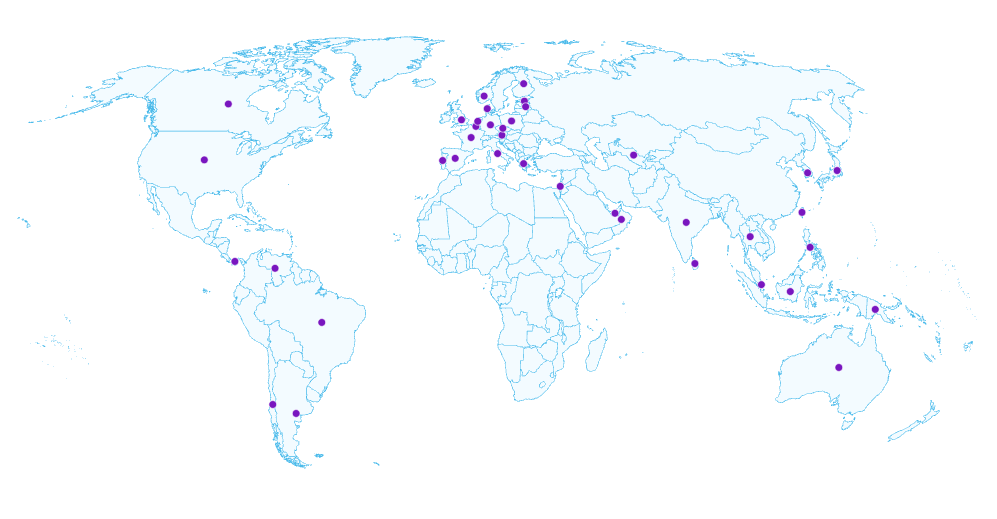
Get seamless, compliant, and secure access to research-ready RWD to support evidence generation across R&D and commercialization activities. Rich mastered data sets that include harmonized and integrated granular patient data provide the reliable foundation you need for discussions with payers, regulators, and other key stakeholders across the entire value chain.
Explore extensive curated datasets that include clinical data, EMRs, eCRFs, labs, multi-omics, imaging data and more across oncology, neurology, rare diseases, immunology, and cardiology. And, we can easily integrate your own data to further enrich your analysis.
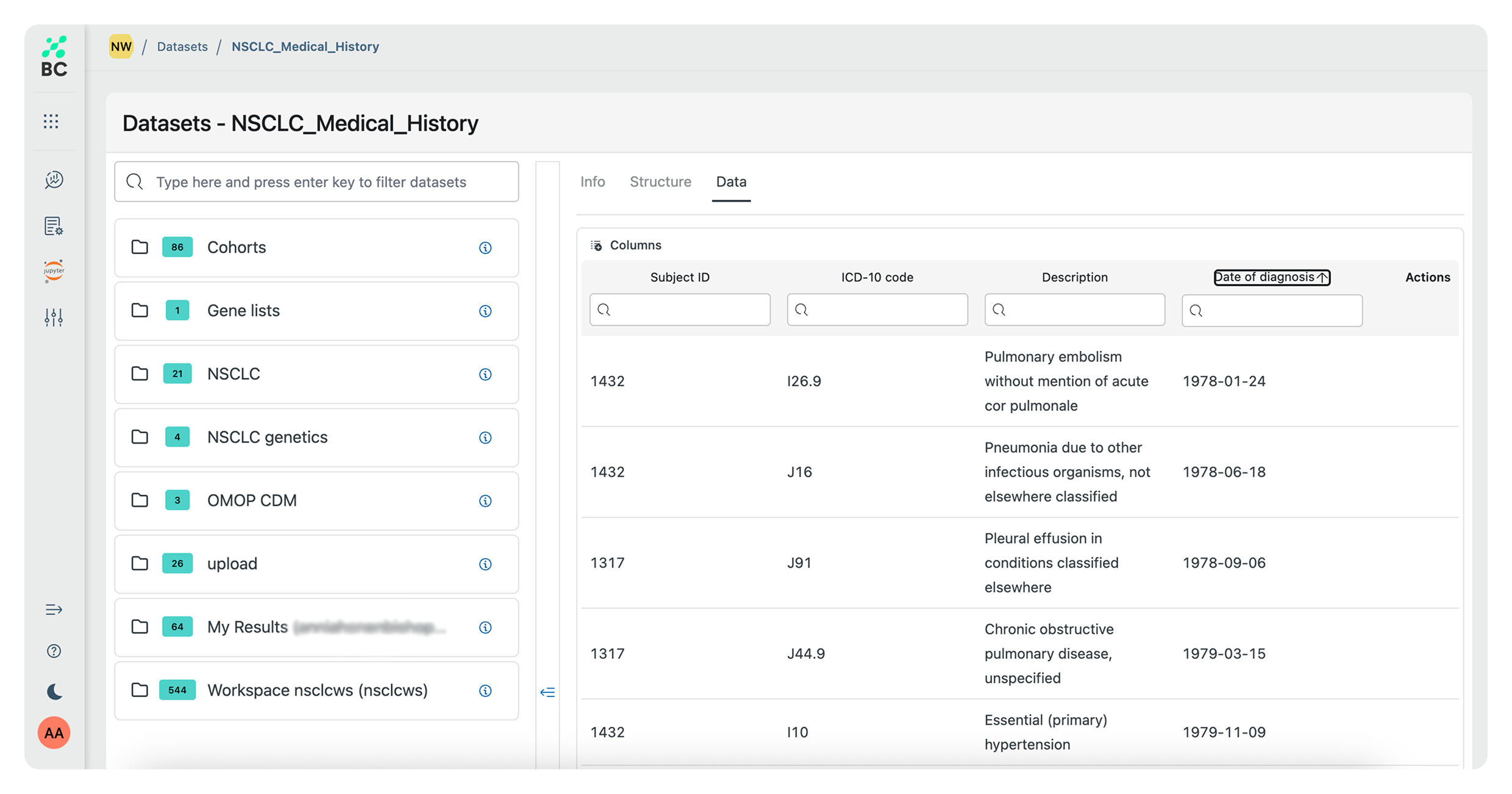
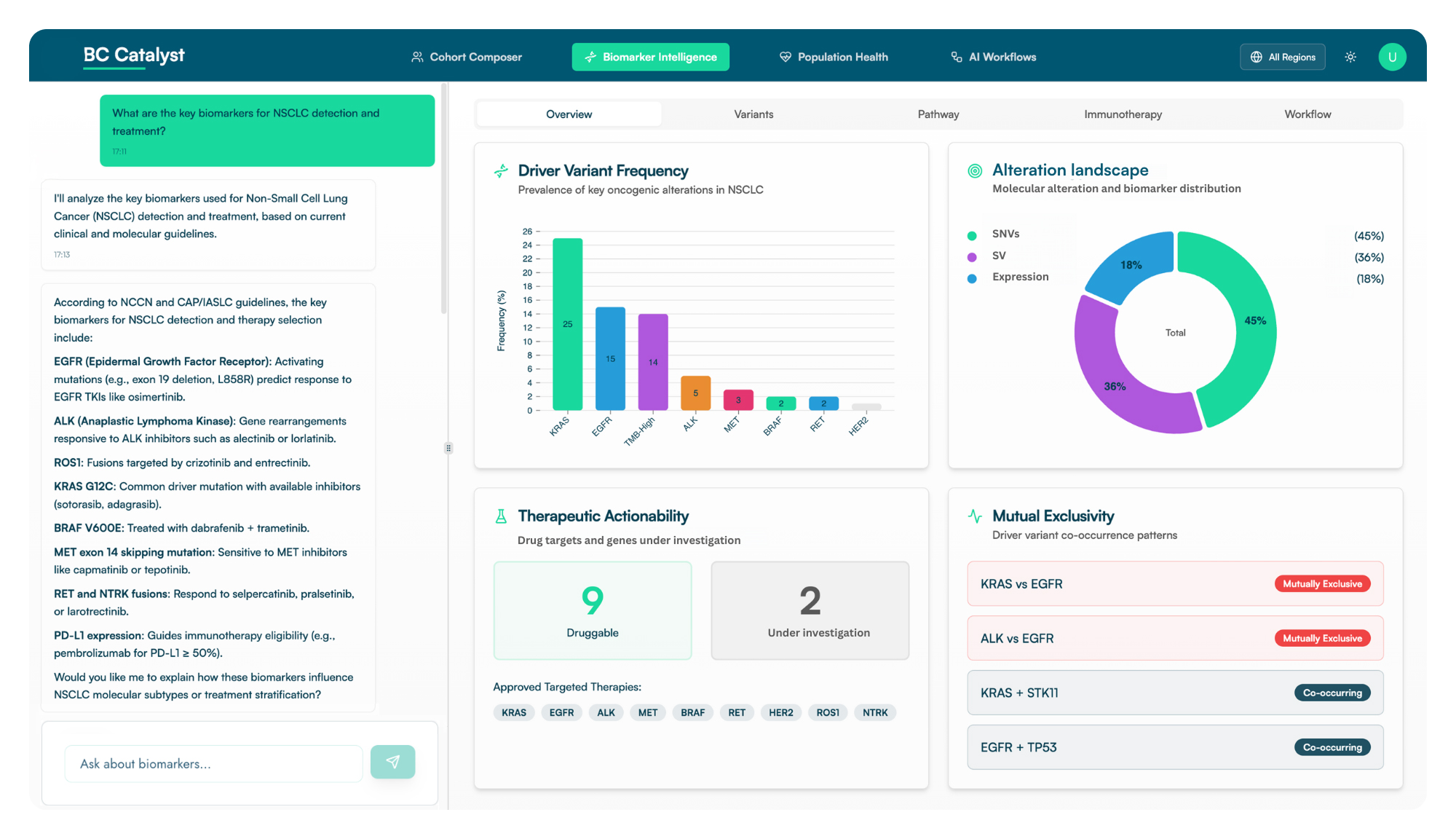
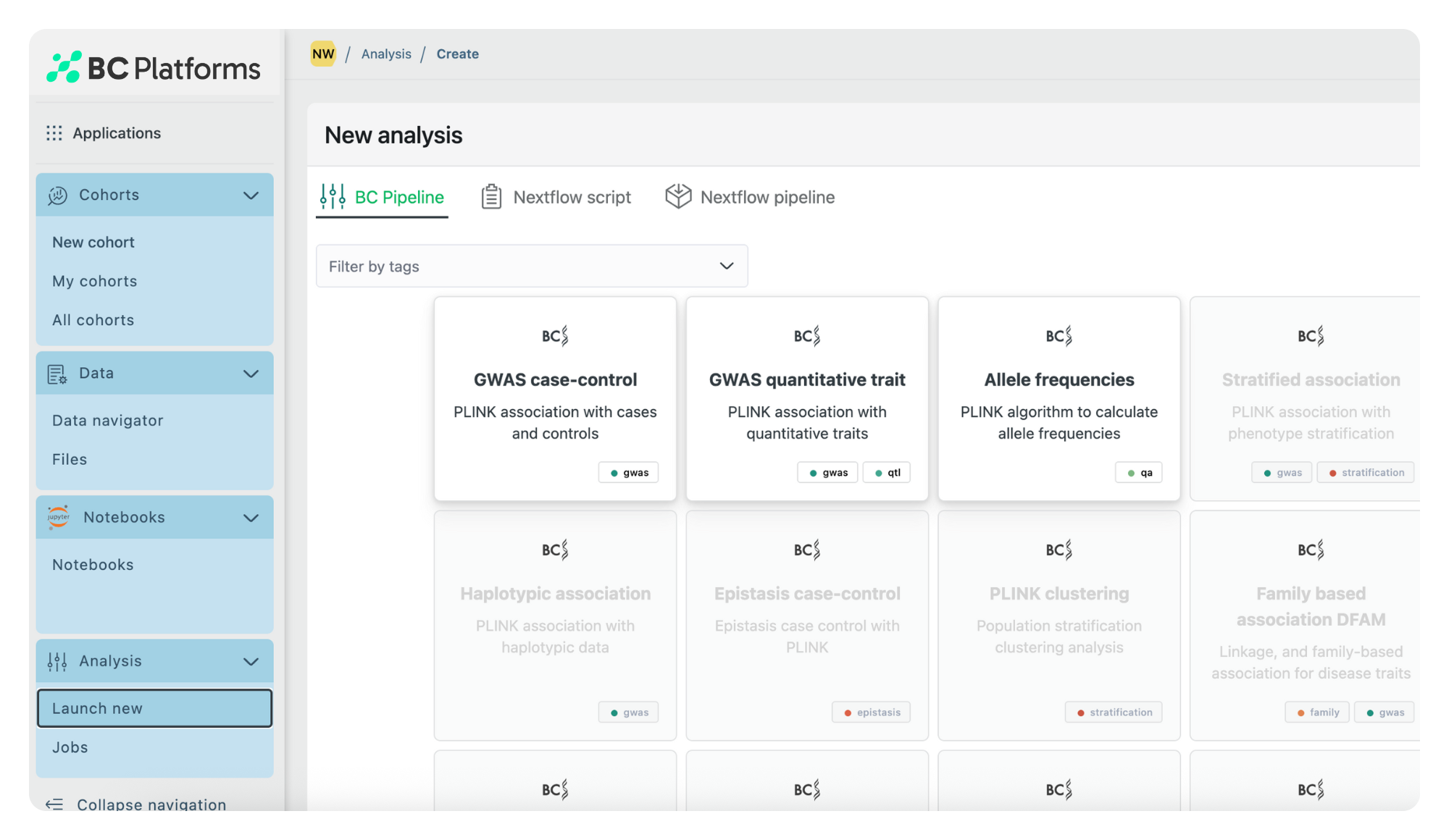
Quickly identify patient cohorts, select biomarkers, and analyze standard of care patterns with BC Catalyst, a prompt-based precision medicine platform that makes complex genomic and real-world clinical data actionable across the entire drug lifecycle.
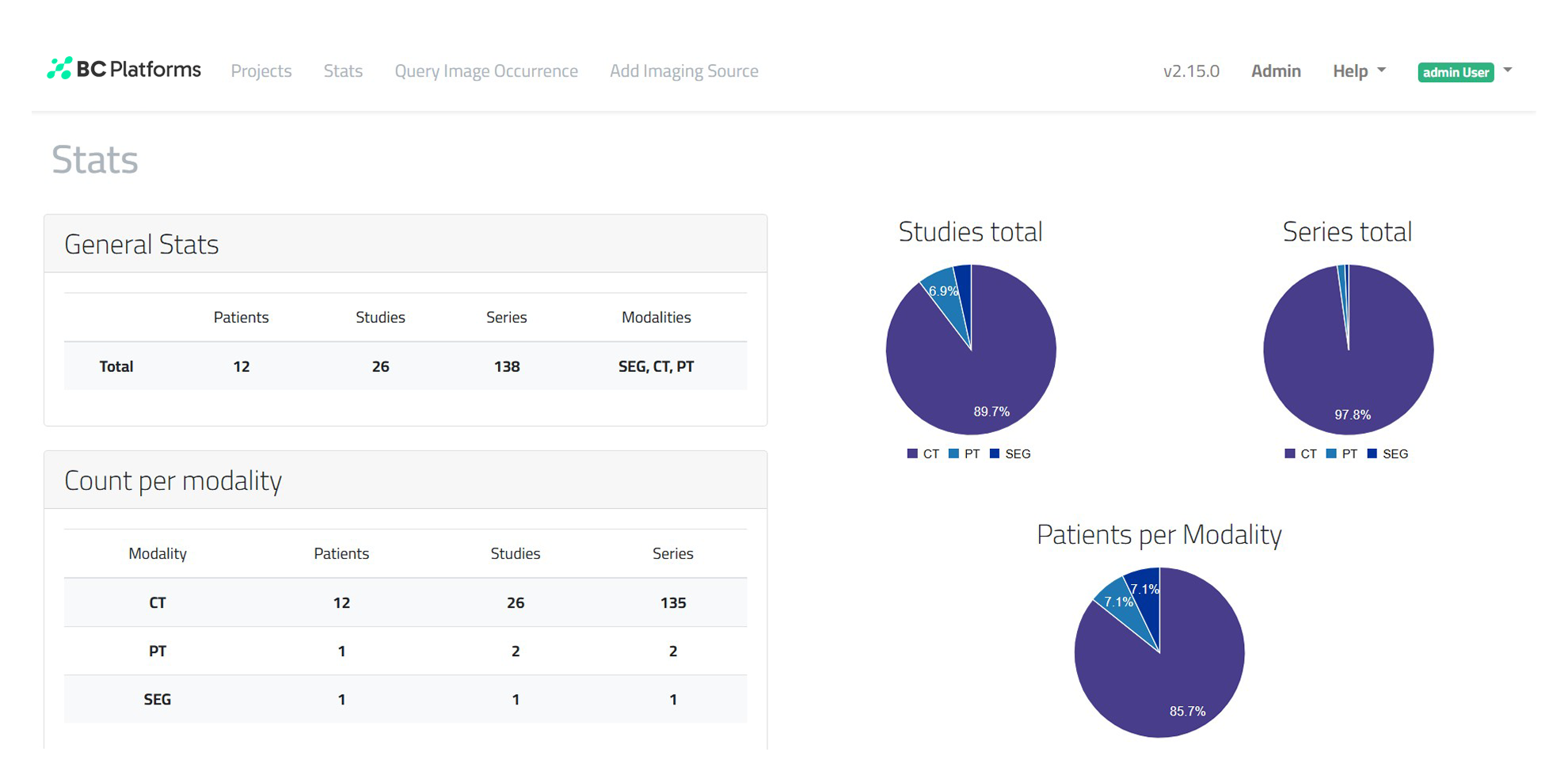
Get standardized, large-scale imaging datasets that are research-ready and anonymized. BC Image accelerates time-to-data and enables advanced, AI-driven analytics for drug development and precision medicine.
Have confidence that data access is well-managed, secure and in compliance with governance standards. Commission analyses from our experts or conduct your own in a built-for-purpose trusted research environment – federated options available.
Get expert support for your trial planning and execution needs from our Scientific Services team, including protocol development and statistical analysis plans (SAP), data science services spanning epidemiology, biostatistics and programming, and medical and regulatory report writing.




As experts in data management and stewardship, we bring proven technology, tools, and experience for processing, analyzing, and sharing sensitive data. Our data mastering platform, BC Unify, transforms and anonymizes multi-modal healthcare data — both structured and unstructured — into harmonized, standardized, analytics-ready datasets.
BC Mosaic, our trusted research environment, now on AWS Marketplace for secure, AI-powered federated data analysis.
BC Catalyst transforms genomic and real-world data into fast, actionable insights to advance precision medicine at scale.
NVIDIA accelerated computing now available in BC Mosaic TRE, delivering up to 80x faster analytics across multi-modal health data.
Contact us to learn more about our solutions, see a live demo, or talk about how we can support your needs.