
BC Catalyst: Your research starts here
Easily explore complex genomics, clinical data, and other rich information using an intuitive AI-powered platform that supports the entire drug lifecycle.
A 360° view of the precision medicine landscape
BC Catalyst is built on a powerful, proprietary AI-driven foundation that enriches our datasets with public-domain intelligence, delivering a 360° view of the precision medicine landscape. Featuring a pharma-grade UX, BC Catalyst makes actionable insights easy to access and enables fast, confident decision-making.
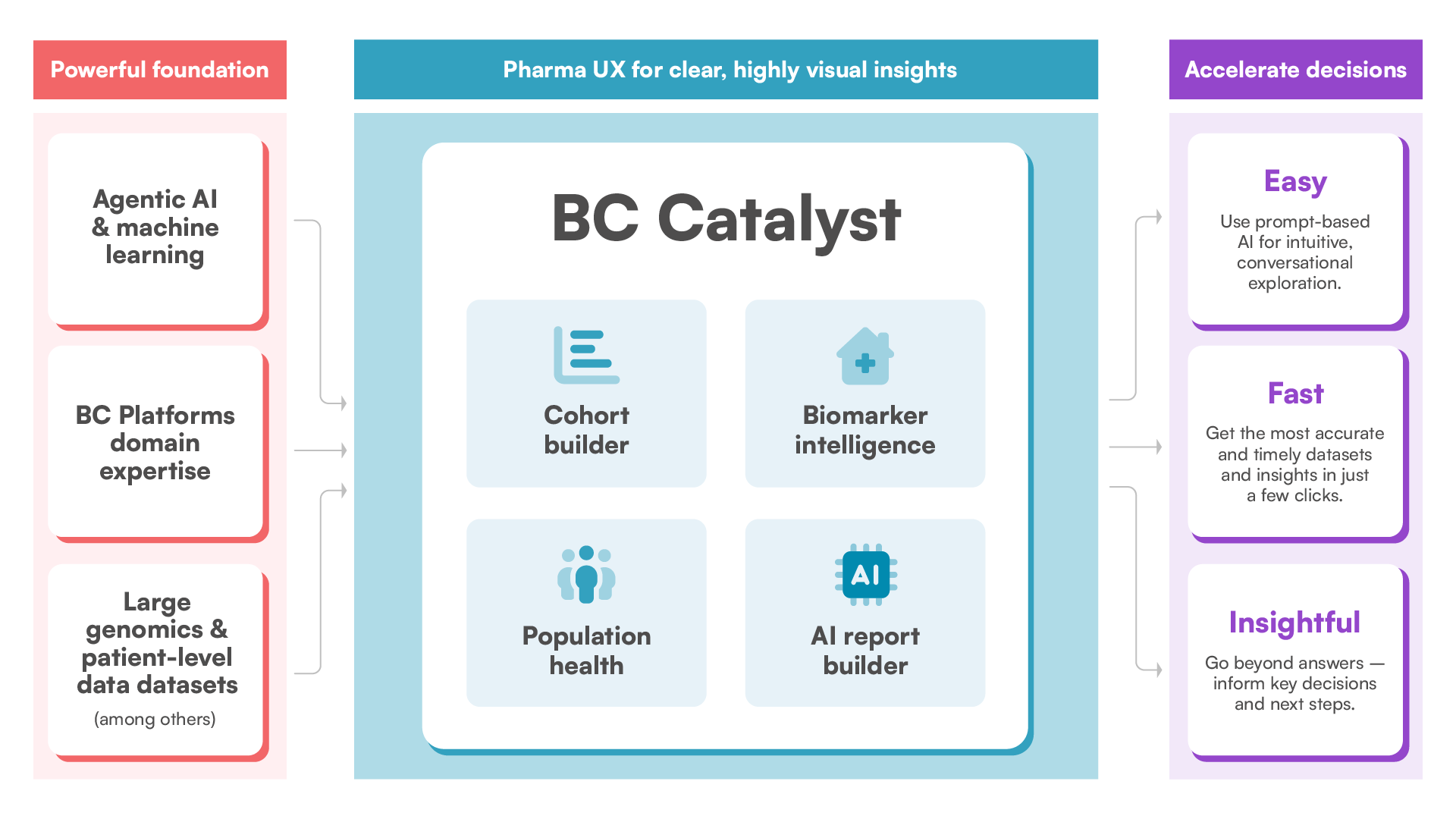
All-in-one cohort building, biomarker intelligence and population health insights
Accelerate your research with BC Catalyst – an intuitive SaaS-based platform featuring prompt-based cohort building, biomarker discovery, and standard of care insights. By combining genomic, clinical, real-world data, and inputs from other public sources in an agentic AI model that continues to learn and grow, BC Catalyst fuels precision medicine strategy, market access, and evidence generation.
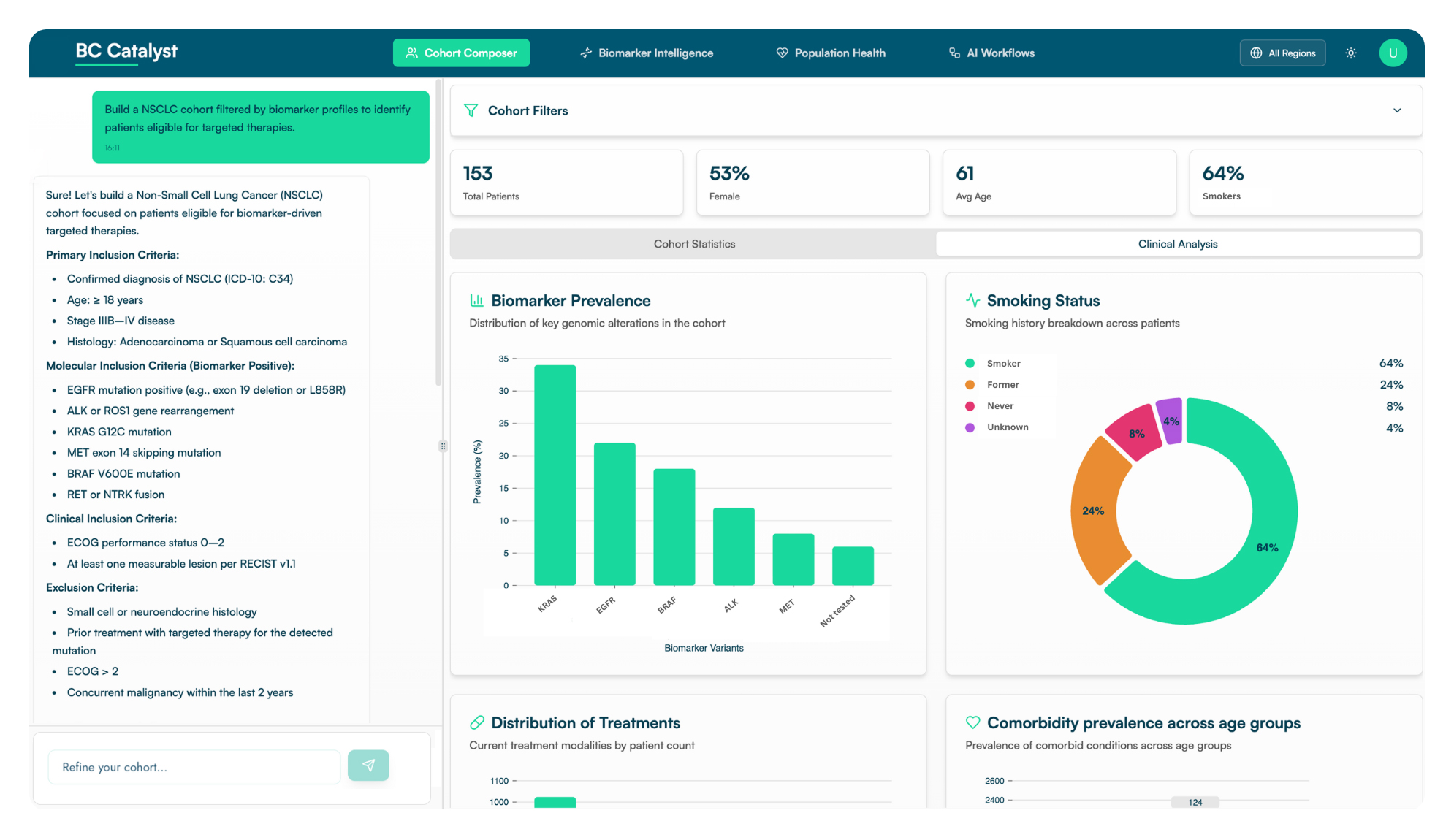
Quickly build and query patient cohorts using harmonized genomic and clinical data. Define, filter, and segment populations across disease areas to support research, trial planning, and commercialization strategies.
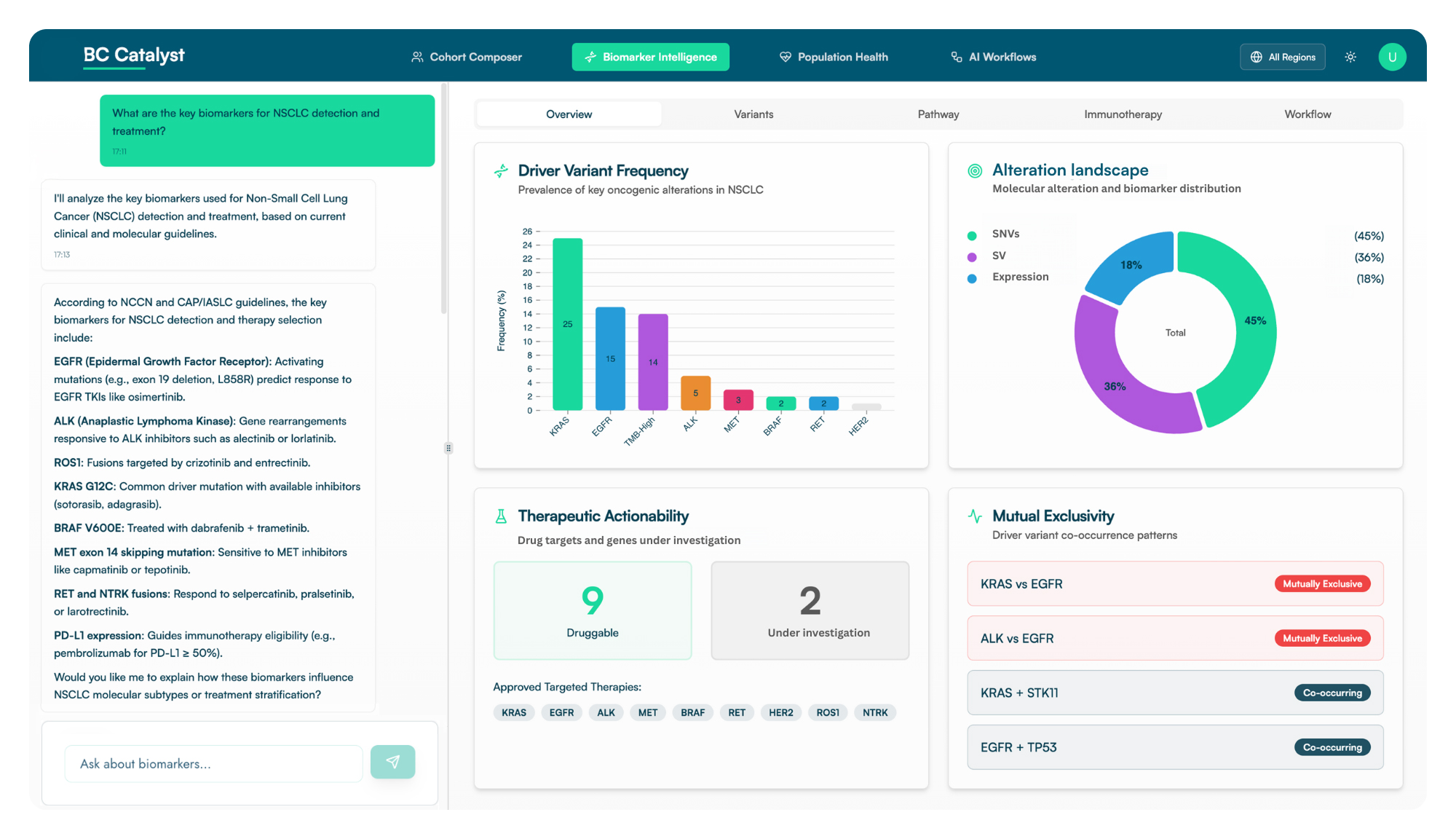
Unlock actionable insights into biomarker-drug-response relationships. Accelerate drug discovery, optimize therapy selection, and predict late-stage failures with advanced analytics and visualizations in just a few clicks.
Reveal population-level health trends, real-world outcomes, and evolving standards of care. Visualize patient journeys, treatment patterns, and market opportunities to inform value-based care and commercial strategies that position your product for success.
Create tailored reports by pulling information from all modules in BC Catalyst. AI-supported workflows and visualizations make it easy to share insights across teams – all in a few clicks and in a model that’s continually learning as you use it.


Powered by BC Unify
BC Catalyst is powered by BC Unify, which acts as the integration engine behind our solutions — ingesting, enriching, and transforming diverse data into a harmonized structure that supports advanced analytics and healthcare research.
Intuitive AI, powerful visuals, effortless adoption
Experience the future of data exploration with BC Catalyst’s agentic AI – prompt-based intelligence and pharma-grade UX that make complex analysis intuitive for every user.
Agentic AI intelligence
Enables intuitive, conversational data exploration for all users through an agentic AI model built specifically for life sciences by our domain experts.
Pharma designed UX
Integrates data, compliance, and usability for translational pharma teams to save time, provide peace of mind, and simplify initial analysis.
Advanced visualizations
Creates an array of data visualizations such as bar charts, pie charts, Sankey diagrams, and risk maps for easy sharing and further exploration.
Easy adoption
Supports rapid adoption within and across teams through a web-based platform that’s available via subscription – no installation required.
Why choose BC Catalyst?
Accelerate drug development
Reduce time-to-market through optimized target identification and patient stratification.
Enhance commercial performance
Increase treatment success rates through precision patient matching.
Mitigate
risks
Minimize late-stage failures and safety concerns through predictive analytics.
Gain market
leadership
Establish a competitive advantage in the rapidly expanding precision medicine market.
Curated knowledge accelerates speed to insight
Multiple use cases across the drug development lifecycle
BC Catalyst delivers significant value to teams across your entire organization – from early-stage discovery through clinical development, product launch and beyond.
-
Drug Discovery
- Biomarker identification
- Drug target discovery
- Disease mechanism elucidation
- Drug repurposing
-
Pre-Clinical / Clinical
- Trial design optimization
- External control arms
- Patient stratification
- Pharmacogenomic analysis
- Real-time cohort monitoring
-
Pre-Launch / Launch
- Regulatory submission support
- Patient recruitment
- Comparative effectiveness studies
- Safety signal detection
- Data harmonization for trials
- Patient journey mapping
- Payer engagement
- Market positioning
- Stakeholder communication
-
Market Access
- HTA submissions
- Regional market tailoring
- Cost-effectiveness analysis
- Predictive modeling
- Patient recontact for studies
-
Post-Launch
- Pharmacovigilance
- Label expansion
- Treatment optimization
- Post-authorization safety studies (PASS)
- Drug repurposing exploration
- Patient-centric outcome tracking
Certified for quality, security, and trust
BC Platforms meets the highest international standards for data protection, product quality, and ethical data governance. Our ISO-certified systems and compliance frameworks protect every stage of the data lifecycle, ensuring partners can innovate confidently and responsibly.
Let’s connect to discuss your needs
Contact us to learn more about our solutions, see a live demo, or talk about how we can support your needs.