EHDS Q&A: Understanding the basics – Part 1
Part 1 of our EHDS Q&A explains why compliance is urgent, outlining key deadlines, enforcement rules, and the financial risks of falling behind.
The Southampton Clinical Trials Unit (SCTU) is a Cancer Research UK core-funded trials unit with expertise in coordinating studies in cancer, early diagnosis, and numerous other areas. It is based within the University of Southampton with offices at the University Hospital Southampton NHS Foundation Trust Southampton General Hospital site.

University Hospital Southampton NHS Foundation Trust is a major National Health Service Trust, serving almost 4 million people across the south of England. It delivers treatments for a wide range of conditions including cancer care.
Every year in the UK, 25,000 people are diagnosed with advanced, inoperable lung cancer. At earlier stages of the disease it can be treated more successfully, reducing death by 19-20%. Unfortunately, at present it often goes undetected. Low-dose CT aid in earlier detection but is labour-intensive and costly.
7,000 people at high risk of lung cancer who were invited to NHS Targeted Lung Health Checks have also taken part in the iDx Lung study, being led by the CRUK-funded Southampton Clinical Trials Unit (SCTU) and the University of Leeds and sponsored by University Hospital Southampton. As well as undergoing a low-dose CT scan as part of the Lung Health Check, participants also gave blood and nasal swab samples which were analysed with simple biological tests. The purpose is to discover whether a low-cost test can also be used for early lung cancer detection as an alternative to CT. BC Platforms’ BC|INSIGHT platform has been delivering rapid insights to the trial, including data harmonisation, management and analysis of research data.
The iDx Lung study has been applying BC|INSIGHT 7, which provides rapid insights through data harmonization, integrated and secure management and analysis of the research data. It delivers an interoperable data architecture, also integrating hospital data to enable secondary health economics analyses. To-date, the study has now completed the recruitment of 7,000 people, with the data to be analysed over the next 3 years.
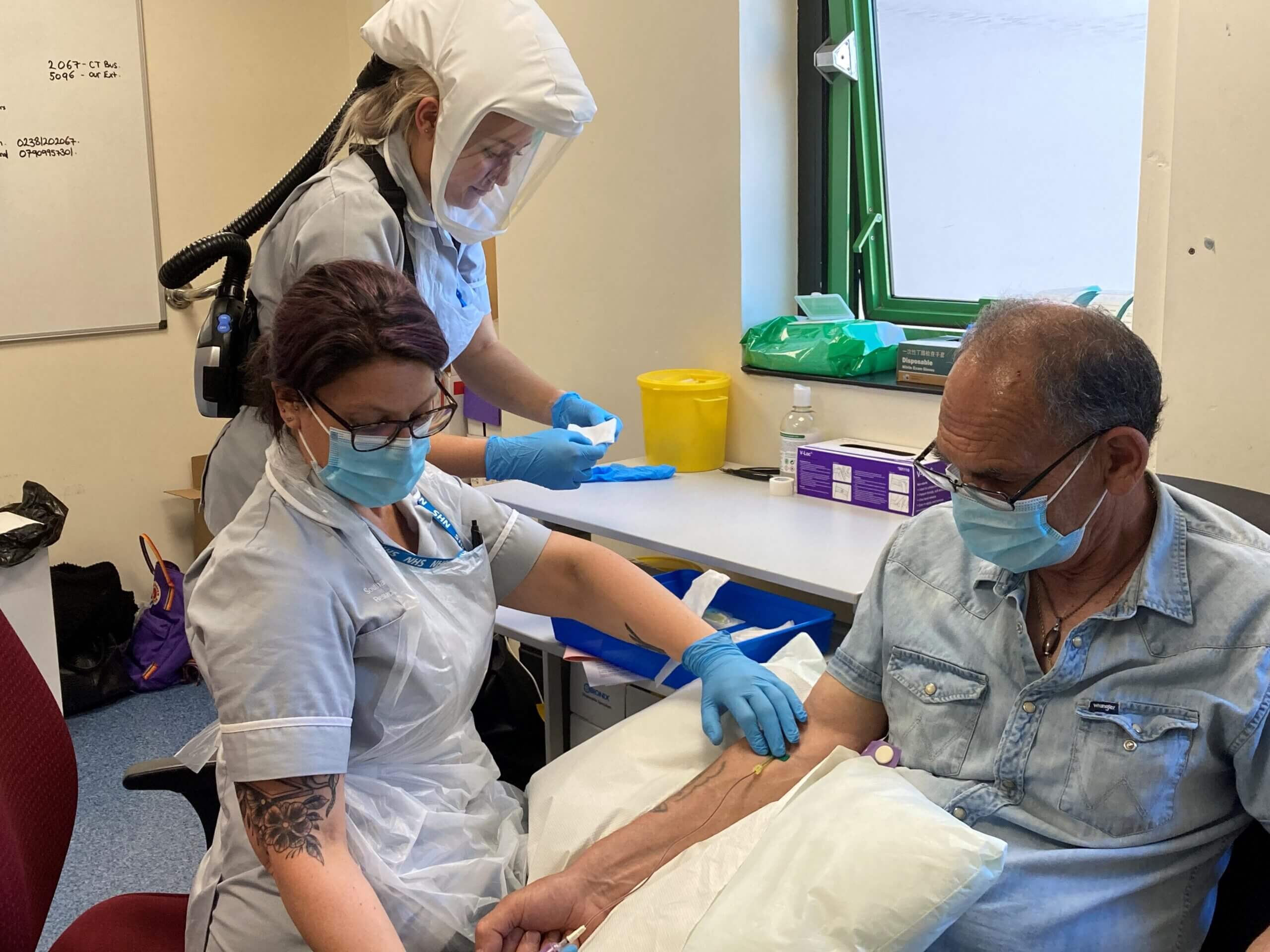
The Southampton Clinical Trials (SCTU) is a UKCRC registered trials unit with over a hundred staff who are experts in study management, quality assurance, data collection and statistical analysis. Working with researchers, clinical teams and industry, SCTU can take a study from initial concept, through funding applications, design and set-up, conduct, to eventual data analysis and publication. The SCTU has a dedicated Early Diagnosis and Translational Research team which is responsible for running the iDx study and specialises in evaluating new ways to detect diseases at an earlier stage and works with industry to use translational samples to further research knowledge and improve future diagnosis and treatment.
BC Platforms has had a long-term, established partnership with the CRUK-funded Southampton Clinical Trials Unit (SCTU), supplying an interoperable data warehouse and analytics platform for translational research. It has delivered the structure, standardisation and normalisation for highly diverse data through a graphical user interface, as well as supporting future patient stratification and precision diagnostic and precision medicine strategies.
We know that if lung cancer is detected earlier, then it can be treated successfully. The iDx Lung study uses some of the latest molecular technology and diagnostic techniques, along with innovative data management and analysis, to try and find better ways to do this and make sure people have the best chance of a cure.
Prof. Peter Johnson, University of Southampton and Chief Investigator of the iDx Lung study
Every year in the UK, 25,000 people are diagnosed with advanced, inoperable lung cancer – the biggest cause of cancer death in the UK and internationally. CT screening is labour-intensive, time-consuming, and costly. A multi-modality approach including blood biomarkers and other surrogate tissue, in addition to CT scanning, might be able to optimise screening for lung cancer, improving early diagnosis and survival.
Additional, secondary outcomes of the study include:
Applying cutting-edge molecular techniques, with NHS England’s Targeted Lung Health Checks programme and the Yorkshire Lung Screening Trial, the local population at high risk of lung cancer has been invited to undergo CT scans to understand how early detection of lung cancer can be improved, including clinical sites in Hampshire (including Southampton), Yorkshire and Greater Manchester. For over 25 years, BC Platforms has been a world leader in life science and healthcare data management and analysis, and provided the trial’s designated software platform, delivering rapid insights through data harmonisation, management and analysis of the research data. It can support a rapid and co-ordinated analysis of data to make informed decisions regarding patients at highest risk of developing lung cancer. The fully persona-driven platform, aligning with the highest data security and regulatory standards, supports large-scale data analysis for insight generation and medical breakthroughs. It also enables linkage with other modular BC Platforms solutions to provide fully secure and highly dynamic federation as evidenced in UK, in amongst others HDR UK’s Co-connect portal, where it supports the federation of millions of patients anonymised data for research across UK.
We adhere to the highest global standards of data privacy and security (GDPR, HIPAA, ISO certifications 13485- Medical device R&D and production and 27001- Data security), and offer a unique and secure trusted research environment solution that enables end users to safely access and utilise data without moving them, safely with state-of-the-art federation – facilitating collaboration and safe data sharing without compromising data security and patient privacy.
Our platform enables the analysis of real-time genetic test data to support clinical decision-making, potentially diagnosing cancer even before it is visible on X-rays. This is another example of our platform being used to more effectively save lives as we facilitate better use of research data across the globe.
BC PLATFORMS
The trial’s data integration, management, and structure has included:
Data can be accessed and analysed through the user’s tool of choice, utilising embedded plugins like notebooks, business intelligence tools, development environments, or SAS services, via BC Dataset Representational State Transfer (REST) services. Population-level end results can then be assessed by the relevant healthcare professionals and help support clinical decision-making and future care and portfolio planning.
It’s important to have platforms that are clinically relevant and meaningful to progress patient outcomes. Ultimately, BC Platforms’ capabilities have stood out in terms of being able to deliver on our requirements and change and adapt to our needs with agility. That’s why our partnership with BC Platforms continues to be valuable.
Prof. James Batchelor, University of Southampton
The iDx-Lung research project is part of the UK Government’s Early Diagnosis Mission to diagnose three-quarters of cancers at an early stage by 2028, so far receiving £3.5million funding from the Government’s Industrial Strategy Challenge Fund (ISCF), as well as £750,000 funding from Cancer Research UK and additional financial support from Yorkshire Cancer Research. This is part of a total investment of £10 million in the programme overall.
With our solution, the research consortium has full control of the data assets. This includes granular data access management and auditing tools for building quality-certified processes, while also integrating hospital data for health economics analyses. We have provided an interoperable data architecture, and its ease-of-use and simple user interface have also enabled and improved collaboration and data sharing with the different organisations in the research consortium. Last year, the trial was awarded the ‘Further, Faster, Together’ Award for industry-academia collaboration at the 2022 Cancer Research Horizons Innovation and Entrepreneurship Awards. 7000 out of a target of 7000 patients have been recruited.